Begin typing your search...
Undoing of the COVID-19 plasma boom
The US invested $800 mn in plasma when the country was desperate for COVID-19 treatments. A year later, the programme has fizzled
Chennai
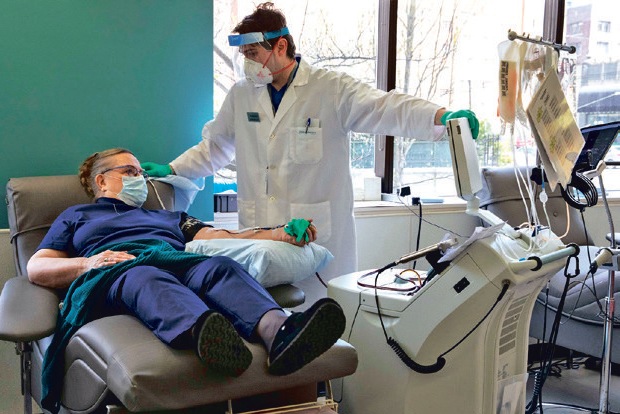
Scott Cohen was on a ventilator struggling for his life with COVID-19 last April when his brothers pleaded with Plainview Hospital on Long Island to infuse him with the blood plasma of a recovered patient. The experimental treatment was hard to get but was gaining attention at a time when doctors had little else. After an online petition drew 18,000 signatures, the hospital gave Cohen, a retired Nassau County medic, an infusion of the pale yellow stuff that some called “liquid gold.” In those terrifying early months of the pandemic, the idea that antibody-rich plasma could save lives took on a life of its own before there was evidence that it worked. The Trump administration, buoyed by proponents at elite medical institutions, seized on plasma as a good-news story at a time when there were not many others. It awarded more than $800 million to entities involved in its collection and administration and put Dr. Anthony Fauci’s face on billboards promoting the treatment.
A coalition of companies and non-profit groups, including the Mayo Clinic, Red Cross and Microsoft, mobilised to urge donations from people who had recovered from COVID-19, enlisting celebrities like Samuel L. Jackson and Dwayne Johnson, the actor known as the Rock. Volunteers, some dressed in superhero capes, showed up to blood banks in droves. Cohen, who later recovered, was one of them. He went on to donate his own plasma 11 times. But by the end of the year, good evidence for convalescent plasma had not materialised, prompting many prestigious medical centers to quietly abandon it. By February, with cases and hospitalisations dropping, demand dipped below what blood banks had stockpiled. In March, the New York Blood Center called Cohen to cancel his 12th appointment. It did not need any more plasma. A year ago, when Americans were dying of COVID at an alarming rate, the federal government made a big bet on plasma. No one knew if the treatment would work, but it seemed biologically plausible and safe, and there was not much else to try. All told, more than 722,000 units of plasma were distributed to hospitals thanks to the federal program, which ends this month.
The government’s bet did not result in a blockbuster treatment for COVID-19, or even a decent one. But it did give the country a real-time education in the pitfalls of testing a medical treatment in the middle of an emergency. Medical science is messy and slow. And when a treatment fails, which is often, it can be difficult for its strongest proponents to let it go. Because the government gave plasma to so many patients outside of a controlled clinical trial, it took a long time to measure its effectiveness. Eventually, studies did emerge to suggest that under the right conditions, plasma might help. But enough evidence has now accumulated to show that the country’s broad, costly plasma campaign had little effect, especially in people whose disease was advanced enough to land them in the hospital.
In interviews, three federal health officials — Dr. Stephen Hahn, the former commissioner of the Food and Drug Administration; Dr. Peter Marks, a top FDA regulator; and Dr. H. Clifford Lane, a clinical director at the National Institutes of Health — acknowledged that the evidence for plasma was limited. “The data are just not that strong, and it makes it makes it hard, I think, to be enthusiastic about seeing it continue to be used,” Lane said. The NIH recently halted an outpatient trial of plasma because of a lack of benefit. Doctors have used the antibodies of recovered patients as treatments for more than a century for diseases including diphtheria, the 1918 flu and Ebola.
So when patients began falling ill with the new coronavirus last year, doctors around the world turned to the old standby. In the United States, two hospitals — Mount Sinai in New York City and Houston Methodist in Texas — administered the first plasma units to COVID-19 patients within hours of each other on March 28. Two developments that month further accelerated plasma’s use. With the help of $66 million in federal funding, the FDA tapped the Mayo Clinic to run an expanded access program for hospitals across the country. And the government agreed to cover the administrative costs of collecting plasma, signing deals with the American Red Cross and America’s Blood Centers.
The news releases announcing those deals got none of the flashy media attention that the billion-dollar contracts for COVID-19 vaccines did when they arrived later in the summer. And the government did not disclose how much it would be investing. That investment turned out to be significant. According to contract records, the U.S. government has paid $647 million to the American Red Cross and America’s Blood Centers since last April.
“The convalescent plasma program was intended to meet an urgent need for a potential therapy early in the pandemic,” a health department spokesperson said in a statement. “When these contracts began, treatments weren’t available for hospitalised COVID-19 patients.” In August, the FDA authorised plasma for emergency use under pressure from former President Donald Trump, who had chastised federal scientists for moving too slowly. At a news conference, Hahn, the agency’s commissioner, substantially exaggerated the data, although he later corrected his remarks following criticism from the scientific community. In a recent interview, he said that Trump’s involvement in the plasma authorisation had made the topic polarising.
“Any discussion one could have about the science and medicine behind it didn’t happen because it became a political issue as opposed to a medical and scientific one,” Hahn said.
In September, the Infectious Diseases Society of America recommended that plasma not be used in hospitalised patients outside of a clinical trial. (On Wednesday, the society restricted its advice further, saying plasma should not be used at all in hospitalised patients.) In January, a highly anticipated trial in Britain was halted early because there was not strong evidence of a benefit in hospitalised patients.
In February, the FDA narrowed the authorisation for plasma so that it applied only to people who were early in the course of their disease or who could not make their own antibodies. Today, several medical centers have largely stopped giving plasma to patients. While scant evidence shows that plasma will help curb the pandemic, a dedicated clutch of researchers at prominent medical institutions continue to focus on the narrow circumstances in which it might work. A clinical trial in Argentina found that giving plasma early to older people reduced the progression of COVID-19. And an analysis of the Mayo Clinic program found that patients who were given plasma with a high concentration of antibodies fared better than those who did not receive the treatment. Still, in March, the NIH halted a trial of plasma in people who were not yet severely ill with COVID-19 because the agency said it was unlikely to help.
The writers are journalists with NYT©2021
The New York Times
Visit news.dtnext.in to explore our interactive epaper!
Download the DT Next app for more exciting features!
Click here for iOS
Click here for Android
Next Story



