
Chennai
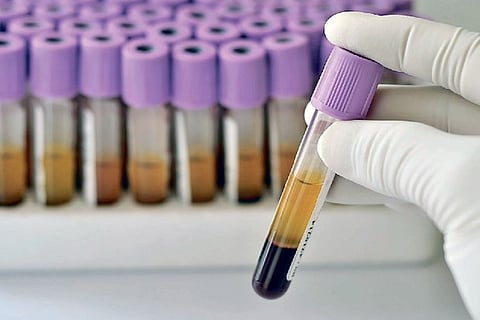
In convalescent plasma therapy, antibodies from the blood of patients who have recovered from the infection are used to treat severely infected patients.
The Drug Control General of India had approved the proposal by the Indian Council of Medical Research (ICMR) to conduct clinical trial.
The one in the works is a multi-centre phase two trial titled “A phase II, open label, randomised controlled trial to assess the safety and efficacy of convalescent plasma to limit COVID-19 associated complications in moderate disease” (PLACID trial).
The PLACID trial protocol has been registered with the Clinical Trial Registry of India (CTRI), with a sample size of 452 persons. However, the number of patients from the State who would be enrolled in the clinical trial is yet to be declared.
Doctors in the COVID-19 ward from the Madras Medical College and Hospital said patients who recovered from COVID-19 will donate plasma on a voluntary basis.
“The paper work for the clinical trial is in progress. The consent of the patients in the moderate disease category, who match the requirements of the clinical trial, will be taken. Plasma donors are being selected and being consented for donation. We are expecting that the groundwork would be over within a week or two and then the trial can start,” said Dr S Subhash, head of Rajiv Gandhi Government General Hospital blood bank.
After ICMR invited letters of interest from hospitals that has the necessary facilities to undertake the study, over 100 institutions had responded.
Of these, 21 have been chosen to take part in the trial including the two institutions from Tamil Nadu. In addition to these two medical colleges, 12 more hospitals from the State have applied for approval to undertake the trial.
Similar efforts to study the efficacy of plasma to treat COVID cases are going on across the world.
Visit news.dtnext.in to explore our interactive epaper!
Download the DT Next app for more exciting features!
Click here for iOS
Click here for Android